About us
We are a company specializing in molecular diagnostics, focused on the development and production of high-quality PCR kits and their components.
Our mission
In the conservative environment of biotechnology companies, we are moving towards simplicity and user-friendliness. We apply our know-how and European quality standards both in innovative products and in modern ordering and shipping methods.
Jan Drápal and Atal Baryal
Every new friendship starts with a greeting, which is why we named our lab HOLA. Hello in Spanish. We believe that even a small biotech company can bring value to laboratories around the world and be successful when compared to established companies.
Our vision
We believe that in the 21st century, quality molecular diagnostics should be available anywhere in the world, for everyone and as a standard.

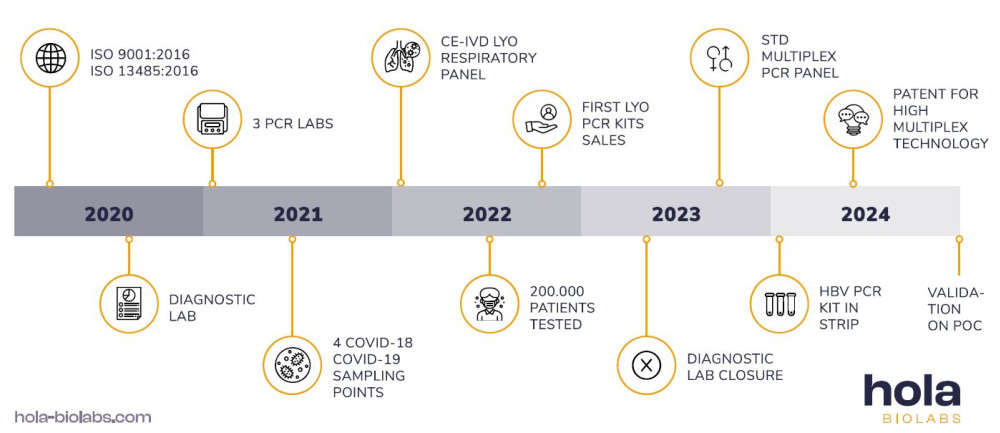
History of Hola Biolabs
The company Hola Biolabs was founded in 2020 in response to the global corona crisis, which exposed the problem of insufficient tools for COVID-19 rapid testing. In the first year, we managed to develop and certify our own PCR kit for the detection of COVID-19.
We also obtained the necessary ISO certificates and became an officially registered non-state medical facility. Within approximately 18 months of operation, we performed over 200,000 tests at our diagnostic facility and became the preferred laboratory for patients and doctors in South Moravia.
Building on our success, in 2022, we developed and certified lyophilized respiratory PCR panel. In 2023, we reached another landmark with the completion of our multiplex lyophilized STD PCR panel and the development of our patent-pending high-multiplex technology.
In 2024, we advanced our innovation by developing PCR kits in the form of single-reaction wells, designed for optional reconstitution with extracted DNA/RNA. This technology was successfully validated on a point-of-care PCR instrument, allowing the simultaneous detection of up to 16 targets.
Major milestones